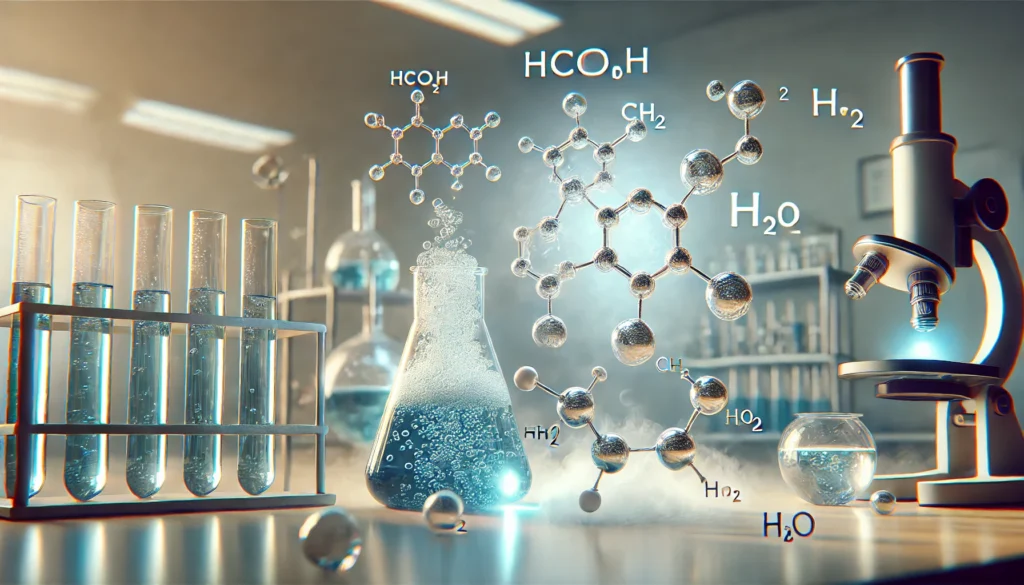
Chemical Structure and Properties of HCOOCH CH2 H2O
The compound HCOOCH CH2 H2O is a fascinating molecule with various uses in chemical industries, especially as a formate ester in aqueous solutions. Understanding the chemical structure and properties of HCOOCH CH2 H2O is essential for industries that utilize esters, solvents, and organic reactions. In this article, we will dive deep into the molecular composition, physical characteristics, synthesis methods, and applications of this compound. Whether you’re a beginner or an experienced chemist, this article will provide you with everything you need to know.
What is HCOOCH CH2 H2O?
HCOOCH CH2 H2O is an ester that consists of a formate group (HCOO), a methylene group (CH2), and water (H2O). Its molecular structure is built on the basic principles of ester chemistry, where an alcohol reacts with a carboxylic acid to form an ester and water. The presence of water alongside the ester group is crucial for various reactions and properties of this compound.
Chemical Structure of HCOOCH CH2 H2O
The chemical structure of HCOOCH CH2 H2O is composed of a formate ester group (HCOO) connected to a methylene group (CH2). The water molecule (H2O) is involved in the molecular arrangement, contributing to its reactivity in certain chemical environments. This simple yet versatile structure allows HCOOCH CH2 H2O to participate in several organic reactions, especially esterifications.
Molecular Composition of HCOOCH CH2 H2O
The molecular composition of HCOOCH CH2 H2O consists of carbon (C), hydrogen (H), oxygen (O), and water molecules. The formate ester group (HCOO) is the core functional unit, while the CH2 group serves as a linker between the ester group and the water molecule. This composition makes the compound an ideal candidate for various applications in organic chemistry and industrial processes.
The Role of Water in HCOOCH CH2 H2O
Water plays a significant role in the chemical behavior of HCOOCH CH2 H2O. In its formation, water is a byproduct of the esterification process. Additionally, water influences the solubility and reactivity of this compound in aqueous environments. It also affects the stability and the polarity of the ester, making it an essential part of its overall chemical properties.
Physical Properties of HCOOCH CH2 H2O
The physical properties of HCOOCH CH2 H2O vary depending on the concentration and environmental conditions. In general, it is a colorless, odorless compound that dissolves easily in water. The presence of the ester group makes it somewhat polar, while the methylene group contributes to its solubility. Its boiling and melting points are relatively low compared to other esters, making it useful in many industrial applications.
How HCOOCH CH2 H2O Forms
HCOOCH CH2 H2O is typically formed through an esterification reaction, where a carboxylic acid (formic acid, HCOOH) reacts with an alcohol (methanol, CH3OH). This reaction produces the ester formate (HCOOCH) and releases water as a byproduct. The presence of water can be both a product and a reactant, depending on the conditions of the reaction.
Chemical Reactions Involving HCOOCH CH2 H2O
The reactivity of HCOOCH CH2 H2O is primarily governed by the ester group. It can participate in hydrolysis reactions, where it reacts with water to revert back to the original alcohol and acid. It can also undergo transesterification, where it exchanges its methanol group with another alcohol. These reactions are important in the synthesis of various other chemicals.
Synthesis of HCOOCH CH2 H2O
The synthesis of HCOOCH CH2 H2O involves a few key steps in organic chemistry. The most common method is esterification, as previously mentioned. The reaction typically requires an acidic catalyst, such as sulfuric acid, to increase the reaction rate and ensure high yield. The reaction occurs under controlled temperatures to prevent excessive loss of water.
Applications of HCOOCH CH2 H2O
HCOOCH CH2 H2O has various industrial and chemical applications. It is widely used as a solvent in chemical processes, particularly in the synthesis of other organic compounds. Additionally, it is employed in some agriculture-related applications, such as pesticides and herbicides. Its unique structure also makes it a useful intermediate in organic synthesis.
Environmental Impact of HCOOCH CH2 H2O
While HCOOCH CH2 H2O is a versatile compound, its environmental impact must be considered. Like many esters, it can degrade over time in the presence of water and certain environmental conditions. However, it is relatively benign compared to more toxic chemicals used in industrial processes. Its biodegradability and low toxicity make it an attractive option for green chemistry initiatives.
HCOOCH CH2 H2O in Green Chemistry
Green chemistry focuses on reducing the environmental impact of chemical processes. The use of HCOOCH CH2 H2O in this field is beneficial due to its relatively low toxicity, ease of synthesis, and biodegradability. It is often employed in the development of more sustainable chemical processes that minimize waste and energy consumption.
HCOOCH CH2 H2O in Organic Synthesis
In organic synthesis, HCOOCH CH2 H2O serves as a valuable reagent in the formation of other esters and organic compounds. Its ability to undergo various chemical reactions, such as transesterification and hydrolysis, makes it a versatile building block in chemical synthesis.
Safety and Handling of HCOOCH CH2 H2O
When handling HCOOCH CH2 H2O, it is important to observe proper safety protocols. While it is not considered highly toxic, it can cause irritation to the skin, eyes, and respiratory system. It should be stored in a cool, dry place away from sources of heat or flame. Proper ventilation should be ensured when working with this compound in a laboratory setting.
How HCOOCH CH2 H2O Interacts with Other Compounds
The interactions of HCOOCH CH2 H2O with other compounds depend largely on the presence of functional groups such as ester and alcohol. It is reactive in the presence of strong acids or bases, which can facilitate hydrolysis and other reactions. It also reacts with other alcohols in transesterification processes to form different esters.
The Future of HCOOCH CH2 H2O in Chemical Industries
As industries continue to focus on sustainability and environmental responsibility, HCOOCH CH2 H2O has great potential. Its relatively benign chemical profile and versatility make it an excellent candidate for new, eco-friendly chemical processes. Ongoing research into its uses in bio-based solvents and as a building block for green chemicals suggests a promising future for this compound.
HCOOCH CH2 H2O in Pharmaceutical Applications
In the pharmaceutical industry, HCOOCH CH2 H2O may be used as a solvent for certain drugs, or as a starting material for the synthesis of drug molecules. Its low toxicity and ability to dissolve various organic compounds make it an attractive option in drug formulation and delivery.
HCOOCH CH2 H2O in Cosmetic Products
Cosmetics manufacturers also look at HCOOCH CH2 H2O for its potential use as a solvent or emollient in skin care products. Its mild properties and ability to blend well with other ingredients make it a candidate for use in creams, lotions, and other formulations.
Synthesis and Reaction Mechanisms of HCOOCH CH2 H2O
The synthesis and reaction mechanisms of HCOOCH CH2 H2O are central to understanding its role in organic chemistry and industrial applications. This compound, which contains an ester group, is highly useful in the formation of other chemicals and in various chemical reactions. In this article, we will explore the synthesis process, the different reaction mechanisms involved, and its practical applications. Whether you’re an organic chemistry student or a professional in the field, this guide will help you understand the intricacies of HCOOCH CH2 H2O.
What is HCOOCH CH2 H2O?
HCOOCH CH2 H2O is an ester compound that consists of a formate group (HCOO), a methylene group (CH2), and a water molecule (H2O). It is commonly formed through the esterification of formic acid and methanol, with water being released as a byproduct. The molecule has various uses in industrial processes, and understanding its formation and reaction pathways is crucial for harnessing its potential.
The Esterification Process in the Synthesis of HCOOCH CH2 H2O
The synthesis of HCOOCH CH2 H2O typically begins with the esterification of formic acid with methanol. This process involves the reaction of the carboxylic acid group in formic acid with the alcohol group in methanol, catalyzed by an acid such as sulfuric acid. During this reaction, water is released as a byproduct, which is removed to drive the reaction to completion.
Key Reaction Mechanisms in the Synthesis of HCOOCH CH2 H2O
The reaction mechanisms involved in the synthesis of HCOOCH CH2 H2O are mainly based on the principles of esterification. The first step is the protonation of the carbonyl oxygen in the formic acid, which makes the carbonyl carbon more electrophilic and susceptible to nucleophilic attack by methanol. This leads to the formation of the ester and the release of water.
Catalysts in the Synthesis of HCOOCH CH2 H2O
The esterification process typically requires the use of a catalyst to speed up the reaction. Sulfuric acid is a common catalyst for this reaction because it helps remove the water by-product, which drives the reaction to completion. Other catalysts, such as Lewis acids, can also be used, but sulfuric acid remains the most effective in this particular synthesis.
Hydrolysis Reactions Involving HCOOCH CH2 H2O
One of the important reactions involving HCOOCH CH2 H2O is hydrolysis, where the ester undergoes a reaction with water to break down into its original components—formic acid and methanol. This reaction can be facilitated by the presence of an acid or base catalyst. Hydrolysis is important in many biological and industrial processes, and understanding this mechanism is key to manipulating the behavior of HCOOCH CH2 H2O.
Transesterification of HCOOCH CH2 H2O
Transesterification is another reaction mechanism involving HCOOCH CH2 H2O, where the ester group reacts with another alcohol, replacing the methanol group with the new alcohol. This reaction is crucial in the production of biodiesel, where vegetable oils or animal fats are transesterified to produce fatty acid esters. The ability of HCOOCH CH2 H2O to undergo transesterification makes it valuable in various chemical and industrial applications.
Dehydration Reactions in the Synthesis of HCOOCH CH2 H2O
Dehydration reactions are also important in the synthesis of HCOOCH CH2 H2O. In these reactions, water is removed from the reaction mixture to drive the formation of the ester. This is especially important in the esterification process, where the presence of water must be minimized to shift the equilibrium toward ester formation. Dehydration agents such as molecular sieves or anhydrous salts can be used to remove water during the reaction.
The Role of Water in the Reaction Mechanisms of HCOOCH CH2 H2O
Water plays a dual role in the chemistry of HCOOCH CH2 H2O. While it is a byproduct in the esterification reaction, it is also involved in hydrolysis reactions, where it helps break down the ester into its components. The presence of water in reaction mixtures can significantly affect the reaction rates and equilibrium positions of esterification and hydrolysis reactions, making it essential to control water content in industrial applications.
Factors Affecting the Synthesis of HCOOCH CH2 H2O
Several factors influence the synthesis of HCOOCH CH2 H2O, including temperature, catalyst choice, and the concentration of reactants. Increasing the temperature generally speeds up the esterification reaction, while the use of excess methanol or formic acid can help drive the reaction to completion. The removal of water is also critical to the success of the synthesis, as water can shift the equilibrium away from ester formation.
The Importance of Reaction Conditions in the Synthesis of HCOOCH CH2 H2O
Controlling reaction conditions is vital when synthesizing HCOOCH CH2 H2O. Temperature, pressure, and the concentration of reactants all play a role in determining the yield and purity of the ester. In some cases, high-pressure conditions are used to increase the solubility of the reactants and accelerate the reaction rate.
Industrial Applications of HCOOCH CH2 H2O Synthesis
The synthesis of HCOOCH CH2 H2O is critical in various industrial applications, including the production of solvents, plasticizers, and other chemicals. The ability to produce HCOOCH CH2 H2O efficiently is essential for industries that rely on this compound in their production processes. Its applications in solvents, coatings, and fuel additives make it a valuable chemical in the global market.
Environmental Considerations in the Synthesis of HCOOCH CH2 H2O
When synthesizing HCOOCH CH2 H2O, environmental considerations must be taken into account. The use of catalysts and the removal of water are important to minimize waste and reduce energy consumption. Additionally, the use of sustainable raw materials in the synthesis process can make the production of HCOOCH CH2 H2O more environmentally friendly.
Future Prospects of HCOOCH CH2 H2O Synthesis
As the demand for more sustainable and efficient chemical processes grows, the synthesis of HCOOCH CH2 H2O is expected to evolve. Researchers are exploring new catalysts and reaction conditions that can further improve the efficiency and environmental footprint of its production. Furthermore, the increasing interest in green chemistry may lead to the development of more eco-friendly methods for synthesizing HCOOCH CH2 H2O.
Analytical Techniques for Characterizing HCOOCH CH2 H2O
Various analytical techniques are used to characterize HCOOCH CH2 H2O and confirm its synthesis. These include spectroscopic methods such as infrared (IR) spectroscopy, nuclear magnetic resonance (NMR), and mass spectrometry (MS). These techniques allow researchers to confirm the molecular structure of HCOOCH CH2 H2O and ensure that the synthesis process has been carried out correctly.
The Role of HCOOCH CH2 H2O in Chemical Synthesis
HCOOCH CH2 H2O is a versatile compound in chemical synthesis. It can be used as an intermediate in the production of other esters, alcohols, and organic compounds. Its ability to undergo various reactions, such as transesterification and hydrolysis, makes it a valuable reagent in the preparation of a wide range of chemicals used in pharmaceuticals, agriculture, and industry.
Safety Considerations in the Synthesis of HCOOCH CH2 H2O
When synthesizing HCOOCH CH2 H2O, safety precautions must be observed. The reactants, especially formic acid and methanol, are corrosive and flammable, requiring proper handling and storage. Adequate ventilation, protective equipment, and fire safety measures should be in place when working with these chemicals in the laboratory or industrial setting.
Applications and Future Prospects of HCOOCH CH2 H2O
The compound HCOOCH CH2 H2O is an ester that is widely used in a variety of industries due to its unique chemical properties. From its role as a solvent in chemical processes to its application in green chemistry, the applications and future prospects of HCOOCH CH2 H2O are vast. In this article, we will delve into the various uses of this compound in industrial processes and explore its potential in future innovations.
What is HCOOCH CH2 H2O?
HCOOCH CH2 H2O is a chemical compound consisting of a formate ester (HCOO), a methylene group (CH2), and a water molecule (H2O). It is formed primarily through esterification reactions involving formic acid and methanol. This compound exhibits a variety of properties that make it a useful intermediate in chemical synthesis and an important component in various industrial applications.
Industrial Applications of HCOOCH CH2 H2O
One of the primary applications of HCOOCH CH2 H2O is in the chemical industry, where it serves as a solvent and reagent in the production of other organic compounds. It is commonly used in the synthesis of esters, alcohols, and other chemicals that are essential in a wide range of industries. Its ability to dissolve both polar and non-polar substances makes it an effective solvent for diverse chemical reactions.
HCOOCH CH2 H2O in Chemical Synthesis
In chemical synthesis, HCOOCH CH2 H2O is used as an intermediate to produce various organic chemicals. It is particularly valuable in the production of formate esters and other alcohol-based compounds. These reactions often involve the esterification process, which makes HCOOCH CH2 H2O a crucial part of the synthetic routes for several important industrial chemicals.
The Role of HCOOCH CH2 H2O in Pharmaceuticals
The applications of HCOOCH CH2 H2O extend to the pharmaceutical industry, where it is used as a solvent and intermediate for drug synthesis. The compound’s chemical properties enable it to effectively dissolve active pharmaceutical ingredients (APIs) and facilitate their incorporation into formulations. As the demand for more sustainable and effective drug delivery systems increases, the role of HCOOCH CH2 H2O is likely to expand.
Environmental Benefits of HCOOCH CH2 H2O
As industries move towards more sustainable practices, HCOCH CH2 H2O is becoming increasingly important in green chemistry. This compound is biodegradable, relatively non-toxic, and can be produced with minimal environmental impact. These properties make it an attractive alternative to more hazardous chemicals used in industrial processes, aligning with global trends toward environmental responsibility and sustainability.
Future Prospects in Green Chemistry
Looking toward the future, the applications and future prospects of HCOCH CH2 H2O in green chemistry are promising. Researchers are investigating its use as a green solvent in various chemical processes, reducing the reliance on traditional solvents that may be harmful to the environment. Its low toxicity and biodegradable nature position it as a key player in the development of eco-friendly chemical processes.
HCOCH CH2 H2O in Agriculture
Another significant area where HCOCH CH2 H2O is used is in the agricultural industry. It is an essential component in the formulation of pesticides and herbicides, where its solvent properties help in the dissolution and distribution of active ingredients. As the agricultural sector moves toward more sustainable and less harmful chemicals, the use of HCOCH CH2 H2O may increase as a safer, more environmentally friendly alternative to conventional chemicals.
Potential for Use in Bio-based Products
The growing trend of bio-based products is likely to influence the future applications of HCOCH CH2 H2O. As demand increases for renewable and sustainable resources, this compound could play a pivotal role in the creation of bio-based chemicals and materials. Researchers are exploring its use in the production of biodegradable plastics and other bio-based products, contributing to a more sustainable future.
HCOCH CH2 H2O in the Automotive Industry
In the automotive industry, HCOCH CH2 H2O is being explored as a component in fuel additives and other chemical processes that improve the performance of engines. Its properties as a solvent and emulsifier allow it to be used in a variety of fuel-related applications, such as enhancing fuel efficiency and reducing emissions. With the rise of green automotive technologies, the role of HCOCH CH2 H2O in this sector is expected to grow.
HCOCH CH2 H2O as a Cleaning Agent
The applications of HCOCH CH2 H2O also extend to cleaning products, where it is used as a mild solvent and cleaning agent. Due to its low toxicity and effectiveness in breaking down oils and greases, it is a safer alternative to harsher chemicals found in traditional cleaning products. This makes it an attractive option for use in household and industrial cleaning applications.
The Role of HCOCH CH2 H2O in Cosmetics
In the cosmetics industry, HCOCH CH2 H2O is used as a solvent and emulsifier in the formulation of creams, lotions, and other personal care products. Its ability to dissolve both water-soluble and oil-soluble ingredients makes it an essential part of many cosmetic formulations. As consumer demand for natural and safe cosmetics continues to rise, HCOCH CH2 H2O will likely see increased use in these products.
HCOCH CH2 H2O in the Food Industry
The applications and future prospects of HCOCH CH2 H2O are not limited to industrial and pharmaceutical sectors. This compound has potential applications in the food industry as well, particularly in food additives, flavorings, and preservatives. As the demand for non-toxic and eco-friendly food processing chemicals increases, the use of HCOCH CH2 H2O in this area is expected to rise.
Exploring the Energy Sector Applications
The energy sector may benefit from the applications of HCOCH CH2 H2O in renewable energy technologies. Researchers are investigating its use in biofuels and energy storage systems. As the world shifts towards renewable energy sources, HCOCH CH2 H2O could serve as an important component in the development of sustainable energy solutions.
Advancements in Biotechnology and HCOCH CH2 H2O
Biotechnology is another area where HCOCH CH2 H2O may play a significant role. Its unique chemical properties make it a useful reagent in various biotechnological applications, including enzyme reactions, fermentation processes, and protein synthesis. With biotechnology advancing rapidly, the potential applications of HCOCH CH2 H2O in this field are vast and will likely continue to expand in the future.
Enhancing Performance in Materials Science
In materials science, HCOCH CH2 H2O is being explored as a potential component in the creation of high-performance materials. Its solvent properties and ability to interact with other organic compounds make it a useful tool in the development of coatings, adhesives, and other materials that require specific chemical characteristics. The future of materials science looks promising with the inclusion of HCOCH CH2 H2O in research and development.
Safety Considerations for Future Use of HCOCH CH2 H2O
As HCOCH CH2 H2O continues to be used in various industries, safety will remain a critical consideration. While the compound is relatively non-toxic compared to other industrial chemicals, it is important to handle it with care and follow appropriate safety protocols. Ongoing research into its safety profile will ensure its continued safe use in industrial and commercial applications.
The Future of HCOCH CH2 H2O in Emerging Technologies
With technological advancements in various sectors, the future prospects of HCOCH CH2 H2O are incredibly promising. As new applications emerge in fields like nanotechnology, energy storage, and smart materials, this compound could serve as a critical component in innovative technologies. Its adaptability and versatility make it a key player in shaping the future of modern industries.
Research and Development in HCOCH CH2 H2O
Ongoing research and development into the applications and future prospects of HCOCH CH2 H2O will uncover new and innovative ways to use this compound. As new technologies and industrial processes evolve, the role of HCOCH CH2 H2O is expected to expand, contributing to a wide range of sectors including pharmaceuticals, green chemistry, and sustainable materials development.
Conclusion: Embracing the Future of HCOCH CH2 H2O
The applications and future prospects of HCOCH CH2 H2O are vast and diverse, making it an essential compound in many industries. As research continues and sustainability becomes a greater priority, the potential for this compound to drive innovation and contribute to eco-friendly solutions is enormous. By understanding its current uses and exploring its future applications, industries can unlock the full potential of HCOCH CH2 H2O in the years to come.
Read More: The Future of Luuxly.com – What’s Next for the Online Shopping Giant